Pepcid as a coronavirus remedy? Trump’s $21-million gamble fizzled
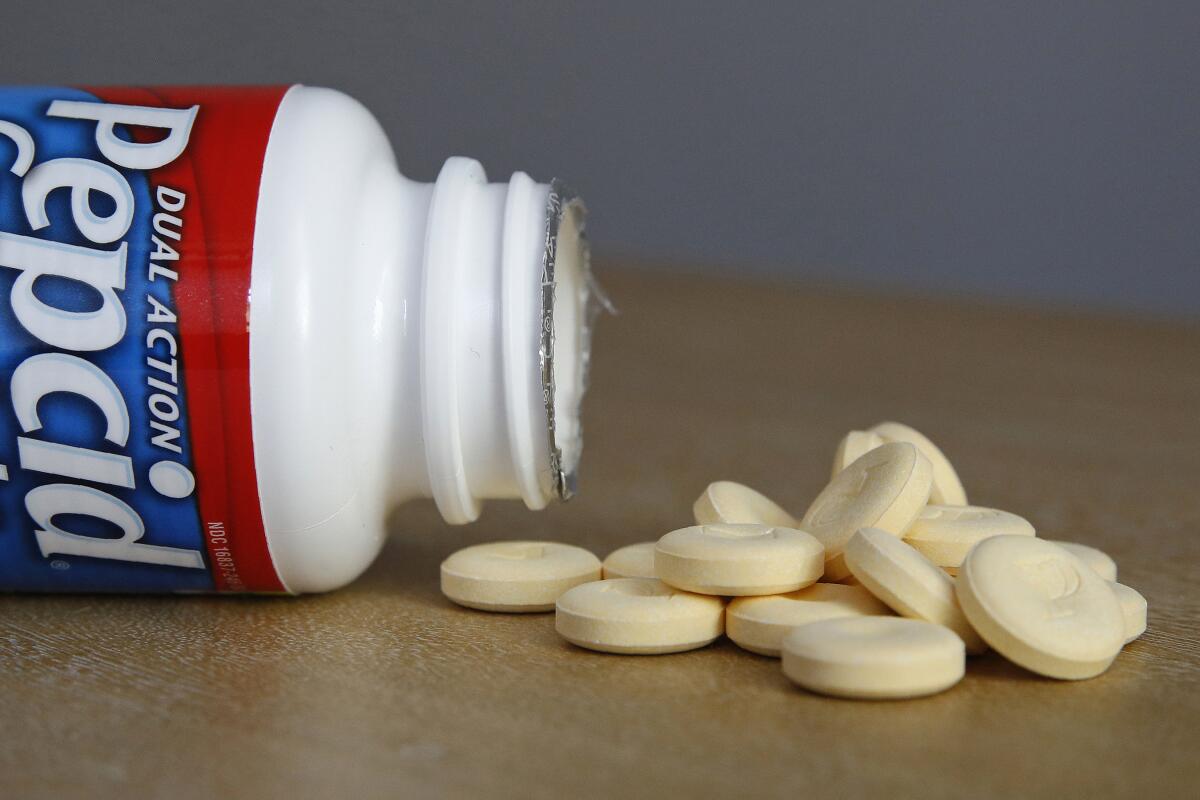
A nearly $21-million government-funded study to see whether a popular over-the-counter heartburn medication could be a COVID-19 remedy has fizzled amid allegations of conflicts of interest and scientific misconduct, according to interviews, a whistleblower complaint and internal government records obtained by the Associated Press.
In mid-April, the Trump administration funded a study of famotidine, the main ingredient in Pepcid, despite a lack of published data or studies to suggest heavy doses would be effective against the novel coronavirus. When government scientists learned of the hastily produced proposal to spend millions in federal funding on the research, they considered it laughable.
Now, the Pepcid project faces an uncertain future. Northwell Health, the New York healthcare provider hired to conduct the testing at its hospitals, put the trial on hold due to a shortage of hospitalized COVID-19 patients in that state. Northwell is partnered with Alchem Laboratories, the Florida-based pharmaceutical company that received the contract.
The Pepcid project underscores what critics describe as the Trump administration’s casual disregard for science and anti-corruption rules — regulations meant to guard against taxpayer dollars going to political cronies or funding projects that aren’t based on rigorous science.
It was harshly criticized by a government whistleblower, Rick Bright. He filed a complaint accusing a senior administration health official of rushing the deal through without the scientific oversight necessary for such a large federal award.
The government had very little data on which to base a funding decision about Pepcid and COVID-19, critics say; there was no high-grade research on famotidine’s coronavirus-fighting potential to underpin a clinical trial involving hundreds of patients.
“The evidence used to support the trial is extremely weak,” said Dr. Steven Nissen, a Cleveland Clinic cardiologist and a frequent adviser to the Food and Drug Administration. “And I’ve been very critical of this approach to the COVID-19 epidemic, which I’ve likened to throwing spaghetti at the wall and seeing what sticks. I consider trials like this one to be largely a waste of time and money.”
The more we stay home, the less the coronavirus spread, according to a new study of 211 U.S. counties that are home to more than half all Americans.
Northwell Health spokesman Matthew Libassi declined to comment on Bright’s whistleblower complaint. “With respect to the famotidine trial, we are confident it is based on sound science,” he said.
Meanwhile, two researchers are locked in a dispute about how the project came to be and who should get credit for the idea.
Dr. Robert Malone, a molecular virologist who was Alchem’s chief medical officer when it won the Pepcid contract, says he was the first to come up with the idea that Pepcid might be effective against COVID-19.
He says he received a call on Jan. 4 from a fellow American doctor, Michael Callahan, working in China. Callahan told him about the new virus that causes severe respiratory illness. Malone then ran the virus’ genetic sequence through computer models designed to find already-approved drugs that might work to thwart the virus, he said. Famotidine turned up as a promising lead.
Three out of four Americans favor requiring people to wear masks outside their homes to help slow the spread of the coronavirus, a new poll has found.
Callahan, who had been working in Wuhan, China, separately claimed he saw data indicating famotidine’s potential in COVID-19 patients, according to promotional materials about a potential Pepcid trial. But Callahan, described by Science magazine as the “first to call attention to the drug in the United States,” never publicly produced any data from his time in Wuhan, according to Malone.
Callahan did not respond to requests for comment.
But Callahan pitched the idea to a key Trump appointee, Dr. Robert Kadlec, assistant secretary for preparedness and response at the Department of Health and Human Services.
On March 20, Kadlec wrote to Northwell’s executive vice president for research, telling him to work with Callahan to prepare a contract proposal and a draft budget for the Pepcid trial.
Federal pandemic response scientists at HHS’ Biomedical Advanced Research and Development Authority, or BARDA, were shut out of these early conversations over famotidine. Rick Bright, BARDA’s director at the time, would later file a whistleblower complaint alleging unethical conduct by agency leadership, and point to the Pepcid trial as a key example.
“By directing a member of his staff (Callahan) to work as an agent of both the company and the government regarding the proposal, Dr. Kadlec was inviting violations of federal procurement law,” Bright said in his complaint.
Single-day coronavirus infections and total hospitalizations also hit new highs in California after a record-high 157 fatalities Wednesday.
Kadlec did not respond to questions about Bright’s allegations, but an HHS spokesperson said senior federal executives often seek expertise both inside and outside of the government. “In that regard, Kadlec is no different,” the department’s spokesperson said in an email.
But two other federal scientists on Bright’s team shared his worries that Callahan’s involvement appeared to be a conflict of interest. Several of them initially saw the Pepcid proposal as a joke; the request was based purely on anecdotal evidence for a trial that would cost millions and take months.
Their concerns were ignored, according to Bright’s complaint and government records. Kadlec oversees Bright’s agency and wanted the Pepcid contract approved — fast.
Scientists say their experimental coronavirus vaccine has been shown in an early trial to prompt an immune response in hundreds of people.
Soon, Bright was reassigned to a lesser role in government.
In late March, the FDA expressed concerns over the famotidine dosage patients were to receive intravenously but agreed to approve the trial after Northwell said it would reduce it, records show. But a senior agency official said the reduced dosage still pushed the levels “to the limits” when compared with previous clinical tests and toxicology studies in animals.
Still, the fast-moving Pepcid proposal had snared the interest of top leadership at HHS, including Secretary Alex Azar, according to the internal emails. After Callahan and Northwell looped in Azar and other top administration officials, however, FDA approved the trial quickly, internal emails show.
California topped New York in coronavirus cases. What is unknown is if the Golden State is on a path to have its hospitals overwhelmed
Experts who conduct clinical trials said this Pepcid study would not have been funded under ordinary, non-pandemic circumstances
“We don’t have enough small studies to show that this is a drug worth pursuing,” said Dr. George Abraham, chair of the American Board of Internal Medicine’s infectious disease group.
Malone resigned as Alchem’s chief medical officer the week the company got the testing contract. He complained of a difficult work environment, and has since been critical of Callahan and the project.
“The Northwell trial is just a zombie at this point,” Malone said. “Completely irrelevant, except in a negative sense.”
Still, Kadlec said through a spokeswoman he would choose to fund the trial again. “If it could save lives, yes.”
More to Read
Start your day right
Sign up for Essential California for news, features and recommendations from the L.A. Times and beyond in your inbox six days a week.
You may occasionally receive promotional content from the Los Angeles Times.